Medical Devices Vigilance Market Demand: Growth, Share, Value, Size, Industry Analsis and Forecast by 2028
"Medical Devices Vigilance Market Size And Forecast by 2028
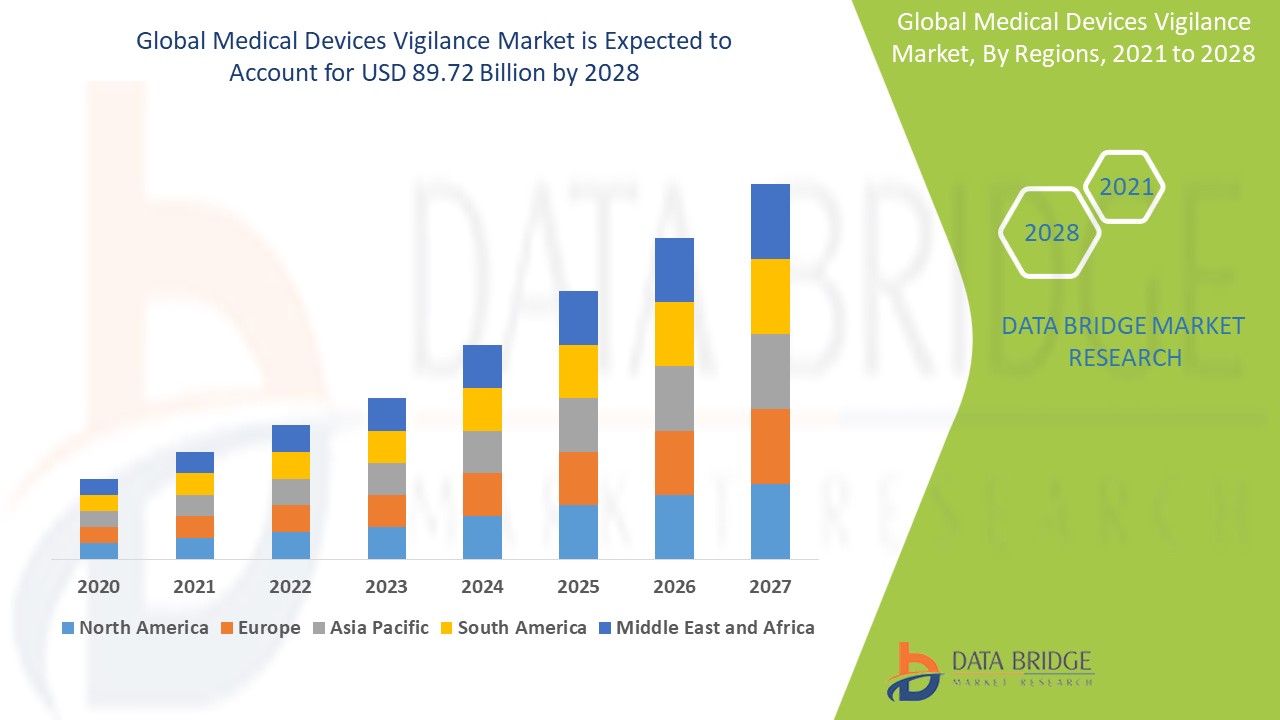
Medical devices vigilance market is expected to gain market growth in the forecast period of 2021 to 2028. Data Bridge Market Research analyses the market to account to USD 89.72 billion by 2028 growing at a CAGR of 6.89% in the above-mentioned forecast period.
Lastly, the study emphasizes the broader implications of these companies’ contributions to the Medical Devices Vigilance Market growth and evolution. Their strategies, technological advancements, and market influence not only define current industry trends but also set the stage for future developments. By providing a comprehensive overview of the leading players, the report equips stakeholders with critical insights to understand competitive positioning, identify opportunities for collaboration, and develop strategies to thrive in this dynamic industry.
Get a Sample PDF of Report - https://www.databridgemarketresearch.com/request-a-sample/?dbmr=global-medical-devices-vigilance-market
Which are the top companies operating in the Medical Devices Vigilance Market?
The Top 10 Companies in Medical Devices Vigilance Market operating in the Medical Devices Vigilance Market are recognized for their innovation, market leadership, and strong presence across key regions. These companies invest heavily in research and development, driving continuous product innovation to meet evolving customer demands. Their extensive distribution networks, brand reputation, and technological expertise have solidified their positions as industry leaders. Additionally, these top companies are expanding through strategic partnerships, mergers, and acquisitions, enabling them to strengthen their market share and enhance their competitive advantages.
**Segments**
- **By Service Type:** The medical devices vigilance market can be segmented based on service type into outsourced services and in-house services. Outsourced services involve the utilization of third-party vendors for the vigilance activities, offering advantages such as cost-effectiveness and expertise. In-house services, on the other hand, are conducted within the organization itself, providing better control and confidentiality.
- **By Delivery Mode:** Delivery mode segmentation includes cloud-based and on-premise solutions. Cloud-based solutions are gaining traction due to their scalability and flexibility, allowing easy access to vigilance data anytime, anywhere. On-premise solutions, though traditional, are still preferred by some organizations for greater data control.
- **By End-User:** The end-user segmentation of the medical devices vigilance market covers hospitals, clinics, contract research organizations (CROs), and others. Hospitals are major end-users due to the large volume of devices used, requiring vigilant monitoring for safety and performance. Clinics and CROs also play a significant role in ensuring safe medical device usage.
**Market Players**
- **Johnson & Johnson Services, Inc.:** A renowned player in the medical devices industry, Johnson & Johnson offers a range of vigilance services to ensure the safety and efficacy of its products. With a strong global presence, the company is committed to upholding high vigilance standards.
- **GE Healthcare:** As a prominent provider of medical imaging and information technologies, GE Healthcare incorporates vigilant practices to maintain the quality and safety of its medical devices. The company's focus on innovation and precision aligns with the vigilance market's requirements.
- **Siemens Healthineers:** Siemens Healthineers emphasizes vigilance in its medical devices portfolio to address regulatory standards and enhance patient safety. With cutting-edge technologies and a commitment to excellence, the company is a key player in the vigilant market segment.
The Global Medical Devices Vigilance Market witnesses significant growth attributed to the rising demand for strict regulatory compliance and the increasing emphasis on patient safety. The market is characterized by technological advancements, such as AI-driven vigilance solutions, that streamline reporting and analysis processes. Additionally, the shift towards proactive vigilance strategies, rather than reactive approaches, is driving market expansion. Emerging economies are also contributing to market growth with increased awareness regarding medical device safety standards. Overall, the medical devices vigilance market is poised for continued advancement with key players investing in innovative solutions and regulatory compliance measures.
https://www.databridgemarketresearch.com/reports/global-medical-devices-vigilance-marketThe landscape of the global medical devices vigilance market showcases a dynamic and competitive environment driven by key market players and evolving trends. One of the notable trends shaping the market is the increasing focus on post-market surveillance and vigilance activities to ensure the safety and effectiveness of medical devices throughout their lifecycle. This trend is fueled by regulatory requirements and the growing importance of real-world data in assessing device performance and patient outcomes.
Another significant trend in the medical devices vigilance market is the adoption of advanced technologies such as artificial intelligence, machine learning, and data analytics to enhance vigilance processes. These technologies enable quicker detection of adverse events, trend analysis, and proactive risk management, leading to improved patient safety and regulatory compliance. Integration of these technologies into vigilance systems is expected to revolutionize how incidents are reported, analyzed, and responded to in the medical devices industry.
Furthermore, there is a noticeable shift towards collaborative approaches among stakeholders within the medical devices vigilance ecosystem. Collaboration between manufacturers, regulatory authorities, healthcare providers, and patients is becoming increasingly crucial in fostering a comprehensive and transparent vigilance environment. This multidisciplinary approach facilitates information sharing, risk communication, and coordinated actions to address vigilance issues effectively.
Moreover, market players are investing in comprehensive training programs and education initiatives to enhance vigilance awareness and capabilities across the industry. By promoting a culture of vigilance and compliance, organizations can mitigate risks, improve reporting accuracy, and ultimately enhance patient outcomes. Training programs cover aspects such as adverse event reporting, signal detection, risk assessment, and compliance with regulatory requirements, contributing to a more robust vigilance infrastructure.
Additionally, the global medical devices vigilance market is witnessing a growing emphasis on data integration and interoperability to streamline vigilance processes and enhance decision-making. Integrated vigilance systems that can aggregate data from multiple sources, including electronic health records, post-market surveillance databases, and device registries, are becoming essential for comprehensive risk assessment and signal detection. Interoperable systems enable seamless data exchange and facilitate holistic vigilance management across the healthcare continuum.
In conclusion, the global medical devices vigilance market is evolving rapidly to meet the increasing demands for safety, quality, and compliance in the medical devices sector. By embracing technological advancements, fostering collaboration, investing in training initiatives, and prioritizing data integration, market players are poised to navigate the complexities of vigilance management effectively. This proactive and interdisciplinary approach is essential for ensuring the continued safety and efficacy of medical devices, ultimately benefiting patients, healthcare providers, and regulatory authorities.**Segments**
Global Medical Devices Vigilance Market, By Delivery Mode:
- On-Demand/Cloud Based (SAAS) Delivery Mode
- On-Premises Delivery Mode
Application:
- Diagnostic
- Therapeutic
- Surgical
- Research
End-User:
- Clinical Research Organizations (CROs)
- Original Equipment Manufacturers (OEMs)
- Business Process Outsourcing (BPO)
Country:
- U.S.
- Canada
- Mexico
- Germany
- Italy
- U.K.
- France
- Spain
- Netherlands
- Belgium
- Switzerland
- Turkey
- Russia
- Rest of Europe
- Japan
- China
- India
- South Korea
- Australia
- Singapore
- Malaysia
- Thailand
- Indonesia
- Philippines
- Rest of Asia-Pacific
- Brazil
- Argentina
- Rest of South America
- South Africa
- Saudi Arabia
- U.A.E
- Egypt
- Israel
- Rest of Middle East and Africa
Industry Trends and Forecast to 2028
**Market Players**
The major players covered in the medical devices vigilance market report are:
- AB-Cube
- AssurX, Inc.
- Oracle
- Sarjen Systems Pvt. Ltd.
- Sparta Systems, Inc.
- Xybion Corporation
- ZEINCRO Group
- Arena Solutions, Inc.
- Intel Corporation
- mdiConsultants, Inc.
- EXTEDO
- Freyr
- Greenlight Guru
- Jama Software
- RELX Group plc
- MasterControl, Inc.
- Panacea Pharma Projects Limited
- Smithers
- Laerdal Medical
- Qvigilance
- Among other domestic and global players.
Market share data is available for Global, North America, Europe, Asia-Pacific (APAC), Middle East and Africa (MEA), and South America separately. DBMR analysts understand competitive strengths and provide competitive analysis for each competitor separately.
The global medical devices vigilance market is witnessing substantial growth driven by the increasing emphasis on regulatory compliance and patient safety. The market segmentation based on delivery mode, application, and end-user provides a comprehensive overview of the varying needs and preferences within the industry. Cloud-based delivery modes offer scalability and accessibility, while on-premises solutions cater to organizations prioritizing data control. The applications of vigilance in diagnostics, therapeutics, surgery, and research highlight the diverse areas where heightened monitoring is crucial. End-users such as CROs, OEMs, and BPOs underscore the collaborative efforts required for effective vigilance practices across the healthcare landscape.
The market landscape is populated by key players like Johnson & Johnson, GE Healthcare, and Siemens Healthineers, known for their commitment to vigilance standards and innovation. However, a myriad of other players, both domestic and global, contribute to the competitive environment with their varied offerings in vigilance services. The market players' focus on technological advancements, data integration, and collaboration aligns with the industry trends driving the evolution of vigilance practices. As market players invest in training programs and emphasize the importance of compliance, the overall vigilance infrastructure is strengthened, ultimately benefiting patient outcomes and regulatory compliance. The forecasted trends indicate a continued reliance on advanced technologies and interdisciplinary approaches to navigate the complex landscape of medical devices vigilance effectively.
Explore Further Details about This Research Medical Devices Vigilance Market Report https://www.databridgemarketresearch.com/reports/global-medical-devices-vigilance-market
Key Insights from the Global Medical Devices Vigilance Market :
- Comprehensive Market Overview: The Medical Devices Vigilance Market is expanding rapidly, fueled by technological innovation and increasing global demand.
- Industry Trends and Projections: Automation, sustainability, and digital solutions are key trends, with the market projected to grow at a significant rate.
- Emerging Opportunities: New opportunities are arising in green technologies and personalized solutions, especially in emerging markets.
- Focus on R&D: Companies are heavily investing in R&D to drive innovation, focusing on AI, IoT, and sustainability.
- Leading Player Profiles: Market leaders like Company A and Company B maintain dominance through strong portfolios and extensive networks.
- Market Composition: The market is fragmented, with a mix of established players and emerging startups targeting various segments.
- Revenue Growth: The market is experiencing steady revenue growth, driven by both consumer and commercial demand.
- Commercial Opportunities: Key commercial opportunities lie in expanding into new regions, leveraging digital transformation, and strategic collaborations.
Find Country based languages on reports:
https://www.databridgemarketresearch.com/jp/reports/global-medical-devices-vigilance-market
https://www.databridgemarketresearch.com/zh/reports/global-medical-devices-vigilance-market
https://www.databridgemarketresearch.com/ar/reports/global-medical-devices-vigilance-market
https://www.databridgemarketresearch.com/pt/reports/global-medical-devices-vigilance-market
https://www.databridgemarketresearch.com/de/reports/global-medical-devices-vigilance-market
https://www.databridgemarketresearch.com/fr/reports/global-medical-devices-vigilance-market
https://www.databridgemarketresearch.com/es/reports/global-medical-devices-vigilance-market
https://www.databridgemarketresearch.com/ko/reports/global-medical-devices-vigilance-market
https://www.databridgemarketresearch.com/ru/reports/global-medical-devices-vigilance-market
Data Bridge Market Research:
Contact Us:
Data Bridge Market Research
US: +1 614 591 3140
UK: +44 845 154 9652
APAC: +653 1251 975