United States Fabry Disease Treatment Market: Detailed Competitive Landscape and Growth Forecast to 2030
United States Fabry Disease Treatment Market Outlook
The United States Fabry Disease Treatment Market, valued at USD 492.11 million in 2023, is set to exhibit impressive growth with a projected CAGR of 6.44% through 2029. The increasing prevalence of Fabry disease, a rare genetic disorder, drives the rising demand for innovative therapies. Recent advancements in treatment options, including enzyme replacement therapy (ERT), chaperone therapy, and substrate reduction therapy, have significantly improved patient outcomes and boosted market expansion.
The ongoing shift toward personalized medicine, which tailors treatments to individual genetic profiles, is revolutionizing the Fabry disease landscape. Government initiatives aimed at advancing rare disease research and improving access to orphan drugs further stimulate the market. Policies such as FDA designations for novel therapies incentivize drug development, supporting innovation.
Leading players like Sanofi SA and Takeda Pharmaceutical Co Ltd continue to dominate the market through investments in next-generation therapies and strategic partnerships. Their competitive contributions ensure a growing selection of effective treatment options, enabling improved care for Fabry disease patients nationwide.
Browse over XX market data Figures spread through XX Pages and an in-depth TOC on "United States Fabry Disease Treatment Market.” - https://www.techsciresearch.com/report/united-states-fabry-disease-treatment-market/17292.html
Market Driver Analysis
The United States Fabry Disease Treatment Market is driven by several critical factors propelling its growth and innovation.
One significant driver is the rising prevalence of Fabry disease, a rare genetic disorder affecting approximately 1 in 40,000–60,000 males, with a lesser but still significant prevalence in females due to partial genetic expression. Growing awareness among healthcare professionals and patients has improved the rate of diagnosis, enabling more individuals to access appropriate treatments. Previously undiagnosed cases are increasingly being identified thanks to advancements in genetic testing and public health campaigns.
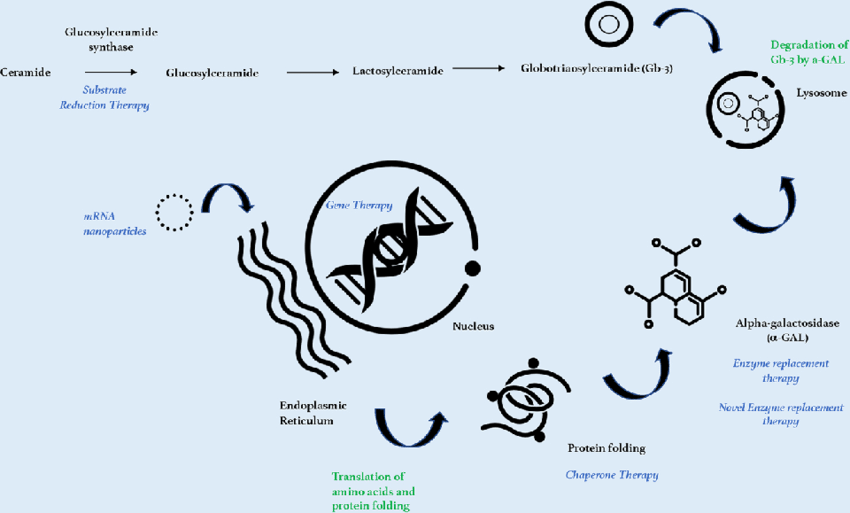
The advancements in treatment options, particularly therapies like enzyme replacement therapy (ERT) and chaperone therapy, have revolutionized the outlook for Fabry disease patients. ERT, including drugs like Agalsidase Beta, effectively replaces deficient enzymes, significantly improving quality of life for patients. Chaperone therapies, such as Migalastat, offer an oral alternative to ERT, enhancing treatment adherence and convenience. These developments are crucial in managing symptoms and preventing long-term organ damage.
Personalized medicine acts as another pivotal driver of market growth. Genetic testing now allows for tailored therapy recommendations, improving patient outcomes and minimizing adverse effects. Healthcare providers are integrating these tools into routine care, ensuring more targeted and effective management of the disease.
Government initiatives and policies aimed at rare disease treatment also significantly impact market growth. For example, the FDA's Orphan Drug Act incentivizes pharmaceutical companies to develop treatments for rare diseases, ensuring continuous innovation and investment in the market. Programs promoting patient registry and clinical trials are further ensuring access to cutting-edge therapies nationwide. These combined efforts contribute to the robust growth trajectory of the United States Fabry Disease Treatment Market.
Download Free Sample Report - https://www.techsciresearch.com/sample-report.aspx?cid=17292
Segmentation Analysis of the United States Fabry Disease Treatment Market
By Treatment Type
- Chaperone Treatment
Chaperone therapy, such as Migalastat, is designed for patients with amenable mutations. This oral therapy provides an alternative to Enzyme Replacement Therapy (ERT), improving enzyme stability and activity. Its convenience enhances patient adherence, making it a critical segment for less invasive management of Fabry disease. - Enzyme Replacement Therapy (ERT)
ERT continues to dominate the treatment landscape. Therapies like Agalsidase Beta are pivotal in replacing the missing enzyme responsible for lipid accumulation. ERT significantly improves patient quality of life and delays organ damage, making it the gold standard for Fabry disease management. - Organ-Specific Treatment
Customized therapies targeting specific manifestations of Fabry disease, such as kidney or cardiac complications, are essential for comprehensive care. These treatments complement core therapies and help manage secondary symptoms to prevent long-term organ failure. - Substrate Reduction Therapy (SRT)
SRT focuses on reducing the production of specific lipids that accumulate due to enzyme deficiency. Though in earlier stages compared to ERT, this innovative approach offers potential for patients who cannot tolerate other treatments, contributing to market expansion.
By Drugs
- Agalsidase Beta
Agalsidase Beta is a leading drug within the ERT segment and is widely trusted for its effectiveness in reducing globotriaosylceramide (GL-3) accumulation. Its established safety profile and efficacy make it a benchmark therapy for Fabry disease care. - Migalastat
Migalastat, an oral chaperone therapy approved for patients with specific genetic mutations, offers flexibility and convenience. Targeted at improving adherence and reducing progression risks, its role in the market continues to grow steadily. - Pipeline Drugs
Innovative drugs in development signal hope for further advancements in Fabry disease care. These pipeline therapies focus on addressing unmet needs, including improved efficacy, alternative mechanisms of action, and minimal side effects, ensuring the market's evolution.
By Route of Administration
- Intravenous (IV)
Intravenous administration dominates the market due to its role in Enzyme Replacement Therapy. Regular IV infusions are standard for delivering essential enzymes directly into the bloodstream, ensuring efficacy in managing Fabry disease symptoms and organ damage prevention. - Oral
Oral administration is gaining momentum with chaperone therapies like Migalastat. Their convenience and non-invasive nature improve patient compliance and broaden treatment accessibility, especially for those living in remote areas or with limited access to regular healthcare facilities.
By Distribution Channel
- Hospital Pharmacies
The hospital pharmacy segment remains dominant, primarily due to the availability of specialized staff and resources required for administering intravenous therapies. These pharmacies cater to patients in need of regular monitoring and long-term disease management. - Retail Pharmacies
Retail pharmacies play a critical role in enhancing accessibility to oral therapies like Migalastat. Their convenience for patients needing routine prescription refills supports improved adherence and market growth. - Online Pharmacies
The growing penetration of online pharmacies provides a convenient option for patients, particularly in rural areas, allowing them easier and timely access to medications. Digital platforms also streamline the process for rare disease patients seeking information and counseling about their therapies.
Each segment uniquely contributes to the United States Fabry Disease Treatment Market by addressing diverse patient needs and advancing the overall standard of care. Together, these components enable robust market growth and highlight the increasing availability of innovative treatment approaches.
Regional Analysis of the United States Fabry Disease Treatment Market
The United States Fabry Disease Treatment Market is significantly shaped by various regional dynamics such as technological progress, regulatory frameworks, innovative investments, and public-private partnerships. These factors combine to create a robust ecosystem for advancing both awareness and treatment of this rare genetic disorder.
Technological Advancements
The United States leads in the development and utilization of cutting-edge therapies for Fabry disease, including enzyme replacement therapies (ERT), chaperone therapies, and emerging substrate reduction treatments. Innovation in gene therapy and personalized medicine is further driving advancements. These technologies ensure more precise and effective care, transforming patient outcomes. Institutions such as the National Institutes of Health (NIH) play a leading role in facilitating the research and development (R&D) of novel treatments, including clinical trials for pipeline drugs with improved safety and efficacy profiles.
Regulatory Environment
The U.S. has fostered a conducive regulatory environment for rare disease medicines, with the FDA’s Orphan Drug Act encouraging pharmaceutical companies to invest in the development of therapies for Fabry disease. Regulatory incentives such as fast-track designations, tax credits, and market exclusivity empower manufacturers and researchers to prioritize innovations in rare disease care. This support helps accelerate drug approvals while ensuring safety and efficacy standards remain uncompromised.
Investment Trends
The market has witnessed substantial investments from both public and private sectors. Top players like Sanofi SA and Takeda Pharmaceutical Co Ltd, alongside emerging companies such as Protalix BioTherapeutics Inc., actively invest in developing next-generation treatments and expanding their local manufacturing facilities. Partnerships between companies and research institutions further fuel these investments. Additionally, patient advocacy groups have contributed by channeling funding into awareness campaigns and research support, ensuring widespread engagement across the healthcare ecosystem.
Presence of Key Players and Research Institutions
The U.S. hub for pharmaceutical R&D, primarily in biopharmaceutical hotspots like Massachusetts, California, and New Jersey, creates a fertile ground for innovation in Fabry disease management. Renowned companies like Amicus Therapeutics and Chiesi Farmaceutici SpA focus on advancing therapies such as oral chaperone treatment and enzyme products. Meanwhile, institutions such as Harvard Medical School and Mayo Clinic collaborate on clinical trials, bolstering advancements in gene therapy and biomarkers for improved diagnosis and monitoring of disease progression.
Prevalence of Fabry Disease
Though rare, Fabry disease impacts a growing number of identified individuals across the U.S. Advancements in genetic testing have significantly improved diagnosis rates, particularly in underserved areas. Previously undiagnosed carriers are now being identified at an increasing pace, ensuring earlier intervention and broader adoption of available therapies. Awareness campaigns led by patient groups and healthcare organizations also educate the public and healthcare providers about the disorder’s symptoms, thereby reducing delays in seeking treatment.
Healthcare Infrastructure
The United States’ well-established healthcare infrastructure enables timely diagnosis and effective disease management. Specialty centers for rare diseases, such as those affiliated with the NIH’s Rare Diseases Clinical Research Network, provide tailored care. Intravenous enzyme therapies are administered at these specialized centers or through home infusion services, ensuring access to critical treatments even in remote regions. Retail and online pharmacies further enhance patient access to oral therapies, particularly improving convenience for long-term treatment adherence.
Collaborations and Initiatives
Collaborative efforts between researchers, pharmaceutical companies, and patient advocacy groups are essential in driving market growth. Programs like the Fabry Registry collect patient data nationwide, supporting research efforts and enabling healthcare providers to make informed treatment decisions. Partnerships between pharmaceutical companies and regional healthcare systems are also streamlining the availability of therapy options, solving accessibility challenges for a geographically diverse patient base.
The United States Fabry Disease Treatment Market is well-configured for sustained growth due to this interconnected framework of regulation, innovation, and infrastructure. This regional robustness ensures improved standards of care and expanding accessibility for Fabry disease patients across the nation.
Primary Catalysts and Hindrances
The United States Fabry Disease Treatment Market is driven by significant catalysts. Advancements in therapies, including enzyme replacement therapy and chaperone treatment, have enhanced patient outcomes and expanded treatment options. Increasing awareness among healthcare providers and patients, supported by public health campaigns and genetic testing improvements, ensures earlier diagnoses and better disease management. Supportive regulatory frameworks, like the FDA's Orphan Drug Act, incentivize pharmaceutical innovations through tax credits and market exclusivity, fueling continued investments in rare disease treatments.
However, hindrances also exist. High treatment costs, often exceeding tens of thousands of dollars annually per patient, limit affordability and accessibility. Additionally, gaps in early diagnosis and a shortage of specialists in remote areas restrict timely intervention and effective management. These challenges underscore the need for enhanced healthcare infrastructure, broader insurance coverage, and targeted awareness programs to bridge these gaps, ensuring balanced market growth.
Download Free Sample Report - https://www.techsciresearch.com/sample-report.aspx?cid=17292
Key Player Analysis of the United States Fabry Disease Treatment Market
- Sanofi SA
- Takeda Pharmaceutical Co Ltd
- Amicus Therapeutics Inc
- ISU Abxis Co Ltd
- JCR Pharmaceuticals Co Ltd
- Protalix BioTherapeutics Inc
- Chiesi Farmaceutici SpA
- Freeline Therapeutics Holdings PLC
- Yuhan Corp
- M6P Therapeutics
Collective Impact on the Market
These players collectively drive the Fabry disease treatment market forward by addressing critical challenges such as adherence, accessibility, and early intervention. Their strategic investments, partnerships, and innovative technologies ensure the continued advancement of treatment modalities, improving patient outcomes and shaping the future of Fabry disease care.
Future Outlook for the United States Fabry Disease Treatment Market
- Technological Advancements: Continued innovation in enzyme replacement therapies and oral chaperone treatments is expected to enhance efficacy while minimizing side effects, improving the overall quality of care.
- Development of New Treatment Options: Pipeline drugs and next-generation therapies, including those with extended half-lives, aim to offer greater convenience and better patient outcomes. These advancements are likely to diversify treatment modalities.
- Emerging Trends in Personalized Medicine: Precision medicine, utilizing genetic testing and biomarkers, will enable tailored therapies specific to patient genotypes, significantly improving effectiveness and reducing risks.
- Gene Therapy Revolution: Gene-editing technologies and vector-based gene delivery systems are poised to provide long-lasting or even curative solutions for Fabry disease, reducing or eliminating the need for lifelong treatment.
- Collaborations and Partnerships: Increased collaboration between pharmaceutical companies and research institutions will drive innovation, accelerate clinical trials, and improve the availability of cutting-edge therapies in the market.
- Regulatory Changes: Anticipated policies promoting orphan drugs and fast-tracking approval processes are expected to incentivize the development of novel therapies, boosting treatment availability.
- Growing Focus on Patient-Centric Care: Holistic approaches to care, including home infusion services for enzyme replacement therapy and digital platforms for patient monitoring, will further enhance patient adherence and convenience.
- Challenges to Address: High treatment costs, gaps in early diagnosis, and logistical obstacles in administering advanced therapies remain key hurdles. Overcoming these will require continued investment in infrastructure and education.
Download Free Sample Report - https://www.techsciresearch.com/sample-report.aspx?cid=17292
LinkedIn – https://www.linkedin.com/pulse/united-states-fabry-disease-treatment-market-cs2mc/
Contact
US -
Techsci Research LLC
420 Lexington Avenue, Suite 300,
New York, United States- 10170
Tel: +13322586602
Email: sales@techsciresearch.com