Healthcare Regulatory Affairs Outsourcing Market Overview: Growth, Share, Value, Size, and Scope , Industry Overview and Forecast to 2032
"Healthcare Regulatory Affairs Outsourcing Market Size And Forecast by 2032
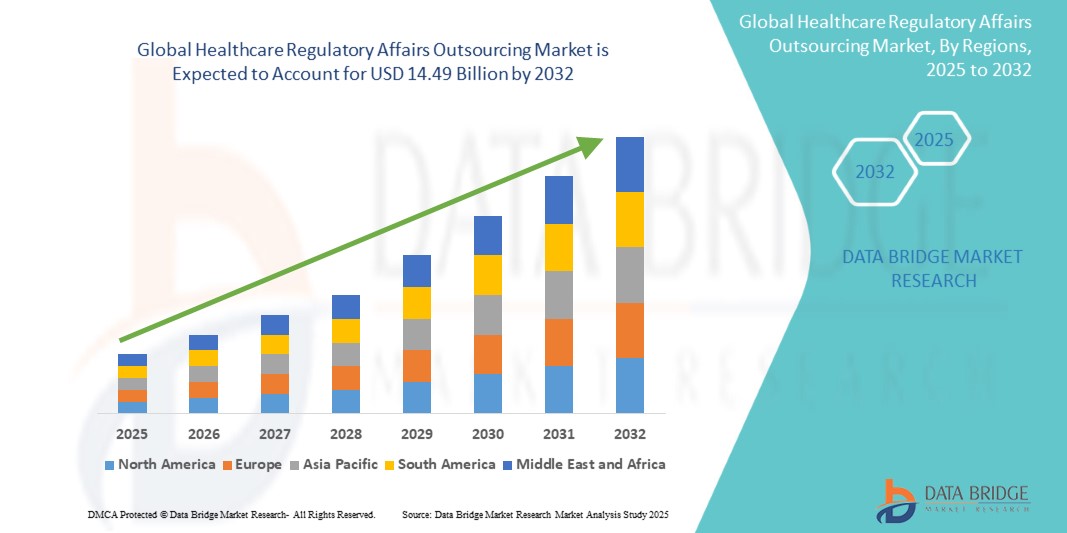
The global Healthcare Regulatory Affairs Outsourcing market size was valued at USD 6.42 billion in 2024 and is projected to reach USD 14.49 billion by 2032, with a CAGR of 10.71% during the forecast period of 2025 to 2032.
Rising demand for Healthcare Regulatory Affairs Outsourcing Market solutions has been a primary driver of market growth, fueled by evolving consumer needs and industry-specific requirements. As companies invest in cutting-edge technologies and expand their reach, the market is set to experience significant revenue growth. This research report delves into the industry’s trends, statistics, and share, offering stakeholders valuable insights into its current performance and future potential.
Get a Sample PDF of Report - https://www.databridgemarketresearch.com/request-a-sample/?dbmr=global-healthcare-regulatory-affairs-outsourcing-market
Nucleus is a secure, cloud-based platform designed to streamline data transfer and management for businesses. Its intuitive interface offers practice administrators and financial managers advanced filtering options, enhancing operational efficiency. By integrating various data sources, Nucleus enables effective prioritization of critical exposures, incorporating business context and threat intelligence to bolster security measures. Additionally, Nucleus supports seamless collaboration among multiple users across different applications, fostering rapid iteration and teamwork. Its deployment flexibility allows installation on-premises or via preferred cloud service providers, ensuring scalability and adaptability to meet diverse organizational needs.
Get More Detail: https://www.databridgemarketresearch.com/nucleus/global-healthcare-regulatory-affairs-outsourcing-market
Which are the top companies operating in the Healthcare Regulatory Affairs Outsourcing Market?
The Top 10 Companies in Healthcare Regulatory Affairs Outsourcing Market are prominent leaders known for their strong influence and significant market share. These include well-established companies which have built a reputation for their high-quality products and services. These companies are recognized for their innovation, customer satisfaction, and ability to adapt to market trends, playing a key role in shaping the growth and direction of the Healthcare Regulatory Affairs Outsourcing Market.
**Segments**
- On the basis of service, the global healthcare regulatory affairs outsourcing market can be segmented into regulatory writing and publishing, clinical trial applications, regulatory consulting, product registration and clinical trial approvals.
- Based on the end-user, this market can be categorized into pharmaceutical industry, medical device industry, biotechnology industry, and other end-users such as food and beverages, cosmetics, and animal health.
**Market Players**
- Some of the key market players in the global healthcare regulatory affairs outsourcing market include ICON plc, PAREXEL International Corporation, Covance Inc., inVentiv Health, Charles River, Medpace, Inc., Accell Clinical Research LLC, Freyr Solutions, Freyr Solutions, ArisGlobal, PRA Health Sciences, Kinapse, ProPharma Group, Promedica International, Weinberg Group Inc., and Covance Inc.
The global healthcare regulatory affairs outsourcing market is witnessing substantial growth and is expected to continue expanding in the forecast period. The increasing complexity of regulatory requirements, coupled with the growing demand for expedited approvals, is driving the market growth. Pharmaceutical companies are increasingly outsourcing their regulatory affairs activities to specialized service providers to ensure compliance with evolving regulations and to expedite the approval process for their products. Additionally, the rise in the number of clinical trials and the increasing outsourcing of drug development activities are further fueling the demand for regulatory affairs outsourcing services.
The regulatory writing and publishing segment is anticipated to witness significant growth due to the increasing emphasis on accurate documentation and a rise in the number of submission approvals. Moreover, the pharmaceutical industry segment is expected to dominate the market share owing to the stringent regulatory requirements imposed by regulatory bodies such as the FDA and EMA. The medical device industry segment is also projected to witness substantial growth due to the rising demand for medical devices and the need for compliance with regulatory standards.
North America currently leads the global healthcare regulatory affairs outsourcing market, followed by Europe. The presence of a well-established pharmaceutical industry, stringent regulatory guidelines, and the presenceThe global healthcare regulatory affairs outsourcing market is experiencing significant growth, driven by factors such as the increasing complexity of regulatory requirements and the escalating demand for accelerated approvals. Pharmaceutical companies are increasingly turning to specialized service providers for regulatory affairs outsourcing to ensure compliance with evolving regulations and to expedite the approval process for their products. This trend is particularly prominent in the pharmaceutical industry, where stringent regulatory requirements imposed by bodies like the FDA and EMA necessitate expert knowledge and resources for navigating the regulatory landscape effectively.
The regulatory writing and publishing segment within the healthcare regulatory affairs outsourcing market is expected to witness substantial growth in the coming years. This growth can be attributed to the emphasis placed on accurate documentation and the rising number of submission approvals required by regulatory authorities. As pharmaceutical companies continue to bring new products to market, the demand for outsourced regulatory writing and publishing services is likely to increase to aid in meeting regulatory standards and timelines effectively.
Another key segment in the healthcare regulatory affairs outsourcing market is the clinical trial applications segment, which plays a crucial role in facilitating the approval process for new drugs and medical devices. With the increasing number of clinical trials being conducted globally, there is a growing need for outsourced support in preparing and submitting trial applications to regulatory authorities. This segment is poised for significant growth as pharmaceutical and biotechnology companies seek efficient ways to navigate the complexities of conducting clinical trials while adhering to regulatory requirements.
In terms of geographical distribution, North America currently commands a leading position in the global healthcare regulatory affairs outsourcing market. The region's dominance can be attributed to factors such as the presence of a well-established pharmaceutical industry, stringent regulatory guidelines, and a strong emphasis on compliance and quality assurance. Europe follows closely behind, driven by similar factors that underscore the importance of regulatory affairs outsourcing services in ensuring compliance and meeting industry standards. As the demand for regulatory expertise and support continues to rise globally, regions like Asia-Pacific are also expected to witness significant growth in the healthcare regulatory affairs outsourcing market as companies seek to leverage opportunities in emerging markets and streamline their**Market Players**
- Accell Clinical Research, LLC (U.S.)
- Charles River Laboratories (U.S.)
- Medwisdom Lifesciences Private Limited (India)
- Indexim International (India)
- Clinilabs Inc (U.S.)
- CRITERIUM, INC. (U.S.)
- Laboratory Corporation of America Holdings (U.S.)
- Cardinal Health (U.S.)
- Freyr (India)
- ICON plc (Ireland)
- IQVIA (U.S.)
- Medpace, Inc (U.S.)
- Parexel International (MA) Corporation (U.S.)
- Promedica International (U.S.)
- Thermo Fisher Scientific Inc (U.S.)
- WuXi AppTec (China)
The global healthcare regulatory affairs outsourcing market is experiencing significant growth driven by factors such as the increasing complexity of regulatory requirements and the escalating demand for accelerated approvals. Pharmaceutical companies are increasingly turning to specialized service providers for regulatory affairs outsourcing to ensure compliance with evolving regulations and expedite the approval process for their products. This trend is particularly prominent in the pharmaceutical industry, where stringent regulatory requirements imposed by bodies like the FDA and EMA necessitate expert knowledge and resources for navigating the regulatory landscape effectively.
The regulatory writing and publishing segment within the healthcare regulatory affairs outsourcing market is expected to witness substantial growth in the coming years. This growth can be attributed to the emphasis placed on accurate documentation and the rising number of submission approvals required by regulatory authorities. As pharmaceutical companies continue to bring
Explore Further Details about This Research Healthcare Regulatory Affairs Outsourcing Market Report https://www.databridgemarketresearch.com/reports/global-healthcare-regulatory-affairs-outsourcing-market
Key Insights from the Global Healthcare Regulatory Affairs Outsourcing Market :
- Comprehensive Market Overview: The Healthcare Regulatory Affairs Outsourcing Market is witnessing rapid growth, fueled by innovation and an increasing shift towards digital solutions.
- Industry Trends and Projections: The market is forecasted to grow at a CAGR of X%, with trends such as automation and sustainability gaining momentum.
- Emerging Opportunities: Growing demand for personalized and green technologies offers emerging business opportunities for new entrants.
- Focus on R&D: Companies are heavily investing in research and development to create next-generation solutions and maintain competitive edges.
- Leading Player Profiles: Dominant players the market with their advanced offerings and strategic expansions.
- Market Composition: The market is a mix of established industry giants and innovative startups, fostering competition and rapid innovation.
- Revenue Growth: Consistent revenue growth is driven by rising consumer demand, technological advancements, and new product introductions.
- Commercial Opportunities: Expanding into untapped regions and investing in emerging technologies presents substantial commercial opportunities for businesses.
Get More Reports:
Artificial Intelligence In Ultrasound Imaging Market Size, Share, and Trends Analysis Report – Industry Overview and Forecast to 2030
Portable Speakers Market Size, Share, and Trends Analysis Report – Industry Overview and Forecast to 2029
Agricultural Acaricides Market Size, Share, and Trends Analysis Report – Industry Overview and Forecast to 2028
Track Loaders for Construction Market Size, Share, and Trends Analysis Report – Industry Overview and Forecast to 2031
Vaping Illness Market Size, Share, and Trends Analysis Report – Industry Overview and Forecast to 2029
Premium Spirits Market Size, Share, and Trends Analysis Report – Industry Overview and Forecast to 2030
Automotive AfterMarket Size, Share, and Trends Analysis Report – Industry Overview and Forecast to 2029
Honey Powder Market Size, Share, and Trends Analysis Report – Industry Overview and Forecast to 2031
Encapsulated Calcium Propionate Market Size, Share, and Trends Analysis Report – Industry Overview and Forecast to 2031
Asia-Pacific Denim jeans Market Size, Share, and Trends Analysis Report – Industry Overview and Forecast to 2030
Europe Fertility Testing Devices Market Size, Share, and Trends Analysis Report – Industry Overview and Forecast to 2029
Europe Operational Technology Market Size, Share, and Trends Analysis Report – Industry Overview and Forecast to 2028
Data Bridge Market Research:
Contact Us:
Data Bridge Market Research
US: +1 614 591 3140
UK: +44 845 154 9652
APAC: +653 1251 975




